KinSight™ Time-Dependent Inhibition Services
Offering you deep kinetic characterization of your lead compounds.
Assessments of the rate of inactivation (kinact), residence time (τ), half-life (t1/2), inactivation efficiency (kinact/KI), and other kinetic parameters are relevant characteristics that are otherwise missed through classical potency determinations with endpoint assays. Understanding a compound’s structure-kinetic relationships (SKR) can greatly improve the effectiveness of SAR campaigns and open avenues for leveraging your inhibitor’s mechanism of action.
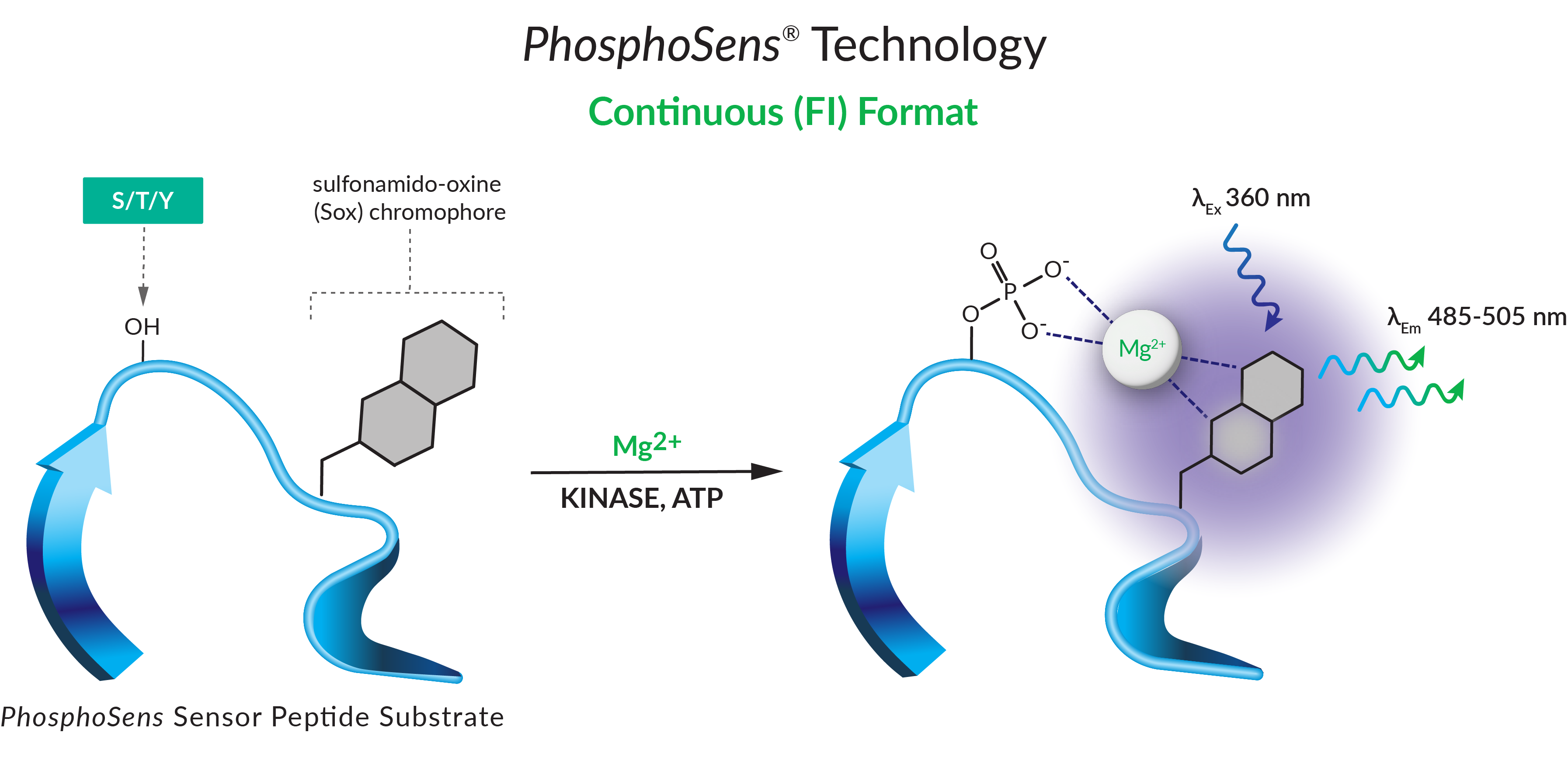
KinSight™ TDI characterization services use our PhosphoSens® direct and continuous assay format to yield an actual reaction rate determined from dozens of data points in a single well. This enables us to provide deep kinetic insights and the kinetic parameters that matter most for your drug discovery.
Why Use a Continuous Assay?
Traditionally, endpoint assays have been used to determine inhibitor potency. However, this format has significant disadvantages in studies involving time-dependent inhibition. An end-point analysis assumes that the initial reaction rate is maintained throughout the forward progress curve, missing important aspects of inhibition modality. Increased interest in slow off-rate and irreversible inhibitors has led to a greater need for a continuous assay format to replace its endpoint counterpart.
Our KinSight TDI services are powered by our novel and proprietary PhosphoSens® Technology — a continuous assay format. Unlike an endpoint assay, it directly quantifies the enzymatic activity of the target by reporting substrate phosphorylation throughout the entire reaction. It yields an actual reaction rate determined from dozens of data points, generating a full progress curve in every well.
Whatever the nature of inhibition—whether it be non-time-dependent, reversible, or irreversible, better understand your lead candidates, inform downstream discovery, and capture inhibitor mechanism of action characteristics with KinSight TDI Services.
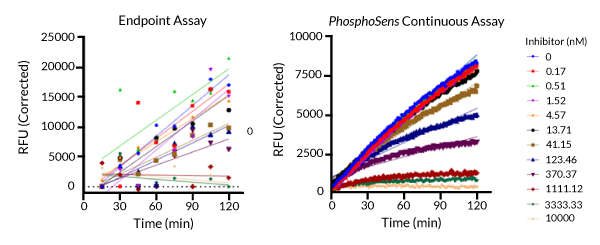
Activity of EGFR tested with Osimertinib (concentration of inhibitor indicated above) using either a commercially available endpoint assay (left) or a PhosphoSens continuous assay (right). With the complete progress curves generated by a continuous assay, you can clearly see TDI evidenced by the non-linear curves. Using the endpoint assay, it is difficult to determine whether there is TDI.
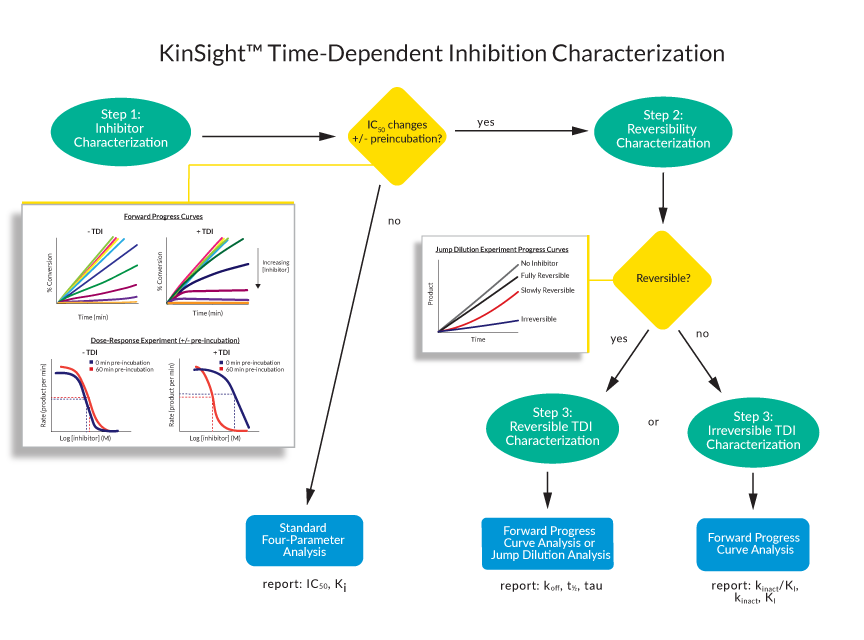
Understanding the KinSight™ TDI Characterization Workflow
Get the most important kinetic parameters you need to understand your compound’s MOA with our validated workflow developed by expert enzymologists. Each stage is designed to deliver specific kinetic insights.
-
STEP 01
Inhibitor Characterization
We start by characterizing your inhibitor using dose response curves with and without pre-incubation of the target enzyme at various inhibitor doses. To recognize TDI, we perform both a qualitative assessment of the progress curve (signal vs time) and quantitative analysis of IC50 (reaction rate as a function inhibitor concentration).
For the qualitative assessment, we visually inspect the linearity of the progress curve—compounds that demonstrate TDI will not appear linear; the slope of the progress curve decreases over time while those that do not demonstrate TDI will have a linear curve throughout.
For the quantitative analysis, we calculate the fold-change in IC50 values with and without pre-incubation of the enzyme with compound. By pre-incubating the enzyme with the compound, the inhibitor can exert any time-dependent effects prior to activity determination, resulting in a more linear progress curve and a change in the IC50.
If we determine there is no observable TDI, we report the IC50 and Ki (if we use ATP Km and binding is competitive with ATP) measurements that are correlated with drug potency. If we see a shift in IC50 values, this is indicative of TDI but doesn’t tell you the whole story. Instead, the kinetic parameters that matter most for your compound’s potency assessment depend on whether it exhibits reversible or irreversible inhibition.
For inhibitors exhibiting TDI, we determine a “proxy IC50”, an IC50 inhibitor concentration derived from the pre-incubation dose response curve that is used as an optimal working inhibitor concentration for the downstream workflow.
-
STEP 02
Reversibility Characterization
Our next step is to identify whether your inhibitor is reversible or irreversible. To determine reversibility, we pre-incubate the enzyme with inhibitor at 10x the proxy IC50 determined in the previous stage followed by a 100x jump dilution experiment. In this assay, the reaction rate of an enzyme bound to a reversible inhibitor would recover nearly to the uninhibited rate, because the inhibitor would quickly dissociate. On the other hand, an irreversible inhibitor would not dissociate, and there would be no recovery of the reaction rate.
Reversible inhibitors will advance to residence time determinations. Irreversible inhibitors advance to determination of kinact/KI, a rate constant describing the efficiency of irreversible bond formation.
-
STEP 03
TDI Characterization
For reversible inhibitors, we perform forward progress curve analysis and jump dilution analysis and report residence time (tau), koff, and t1/2.
For irreversible inhibitors, we perform forward progress curve analysis and report the inactivation efficiency, kinact/KI, and where possible each kinetic parameter separately: kinact, the maximal rate of inactivation, and KI, the kinetic binding constant (depending on whether the global data fit to a 1 or 2 step model, respectively).
These parameters, particularly the inactivation efficiency, are critical to interpret structure-activity relationships and can more effectively translate activity from biochemical assays to the cell than traditional IC50 comparisons. The inactivation efficiency is the best and most-often used parameter to describe the effectiveness of an irreversible inhibitor. Journals are beginning to require kinetic characterization for TDIs and will not accept steady-state IC50 reporting.

The Importance of Continuous Assays for Time Dependent Inhibition
Learn more about the time-dependent inhibition workflow at AssayQuant. Our Sr. Director of Discovery Technologies, Earl May, Ph.D., will walk you through our workflow and give important insights about these kinetic parameters and how they can be used to determine your compounds mechanism of action.
Defining Kinetic Parameters
Ki: Allows for the independent kinetic characterization of the reversible binding of your compound to its target and comparisons across enzymes and substrates. The determination of a Ki requires the measured IC50 in the experiment, an understanding of the MOI of the inhibitor, the Km, and the concentration of active enzyme in the assay.
- kinact: maximal rate of inactivation
- KI: kinetic binding constant, concentration at half maximal rate
Explore Our Other KinSight™ Services
Quantitative Kinome Profiling Services
Compound Testing Services
Custom Assay Development Services
Stay Informed
Want to hear the latest about our technology? Be among the first to learn about our latest products and services.